The Genus Diospyros in New Caledonia
- Main
- Research
- Fieldwork
- People
- Publications
Main
Background
With over 500 species worldwide, Diospyros is one of the largest angiosperm genera. Of the 11 main clades determined by Sutee Duangjai in 2009 (link), three occur in New Caledonia. Of the 31 New Caledonian species, all but one are endemic and one large group (24 species) shows evidence of rapid diversification following long-distance dispersal.
Current Work
Our group is applying molecular analysis of nuclear low copy genes, population genetic techniques (AFLP, RAD sequencing), and cytogenetics to explore evolutionary processes and interspecies interactions in the genus. These methods form the basis of Barbara Turner's doctoral work, here in Vienna.
The goal is to understand the complex processes of speciation among a diverse, but clearly defined species group in an island setting. Research themes include the extent of gene transfer between- and among paleoendemic and neoendemic lineages, the balance between ecological niche separation and genomic incompatibility in forming species boundaries, and molecular dating of the endemic clades.

Diospyros labillardieri

Diospyros pancheri

Diospyros veillonii

Diospyros vieillardii
New Caledonia
Located in the Pacific some 1300 km east of Australia and with a land area of about 19,000 km², this tropical archipelago is one of the world's 34 biodiversity hotspots. 75 % of the flora is endemic, but only small fragments of natural vegetation have survived human habitation.

Research
Phylogenetics

D. cherrieri
Phylogenetics
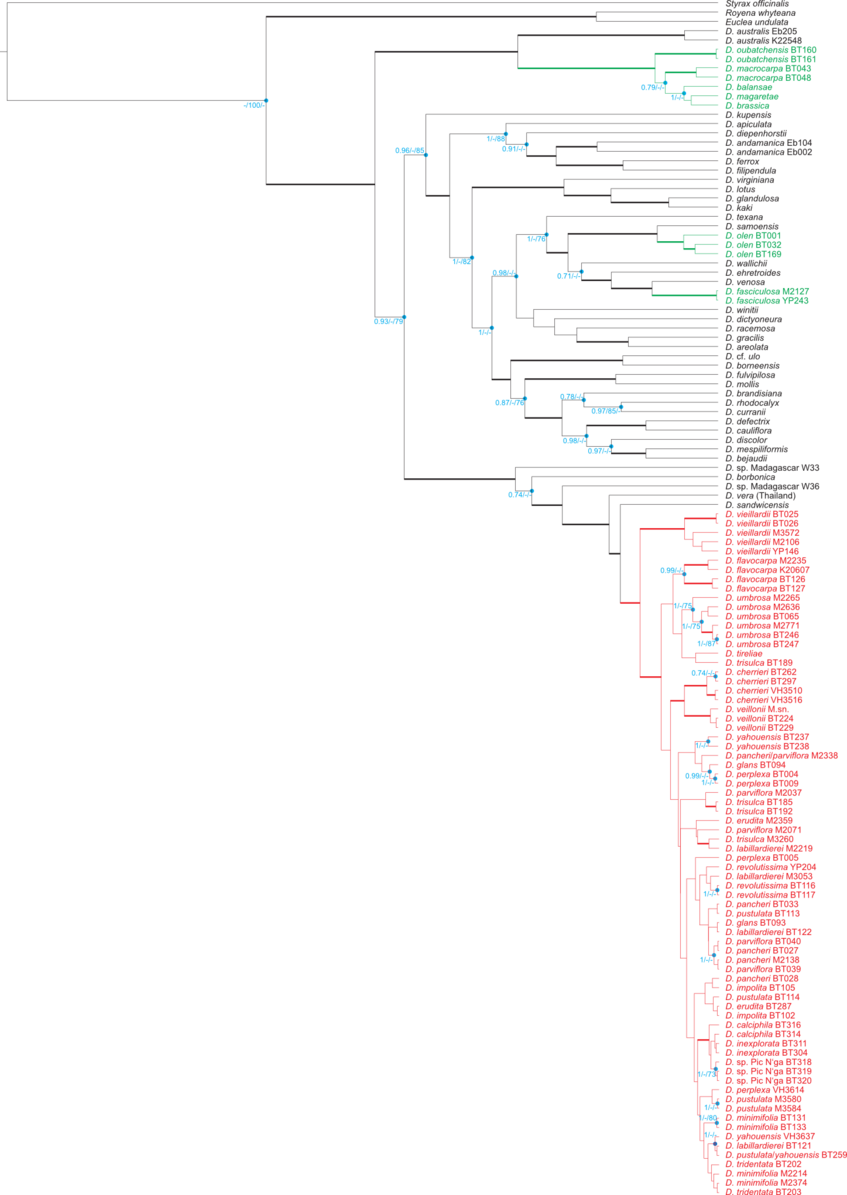
Phylogenetic analysis enables us to understand the origins of New Caledonian Diospyros and their relationship to other members of the genus around the world: How many lineages arrived in New Caledonia, how long ago, and how they have diversified since their arrival.
We have analysed DNA sequences from two low-copy nuclear genes (ncpGS and PHYA) and four plastid loci (atpB, rbcL, trnK–matK and trnS–trnG) using BEAST (Bayesian), PAUP* (maximum parsimony) and RaxML (maximum likelihood).
The figures below are modified from Turner et al. 2013, Molecular Phylogenetics and Evolution. (Right-click to enlarge).
LEGEND:
Left figure: Bayesian maximum clade credibility tree from a combined nuclear & plastid DNA data set. Bold branches have >70 % support from all three analyses; blue dots indicate ≥70 % support from at least one analysis (Bayesian/maximum parsimony/maximum likelihood). New Caledonian taxa are coloured: red represents the rapidly diverging clade III.
Figure in the middle: Chronogram from BEAST, based on a combined nuclear & plastid DNA data set. Ages are given (in million years) for nodes with >0.85 Bayesian posterior probability. Black dots are calibrated nodes. Yellow bars represent the 95 % highest posterior density interval. New Caledonian taxa are coloured: red represents the rapidly diverging clade III.
Right figure: One of 210 equally parsimonious trees from plastid DNA sequences. Clades are named according to Duangjai et al., 2009. Bold branches have more than 70 % support from all three analyses. New Caledonian taxa are coloured: red represents the rapidly diverging clade III.
combined matrix
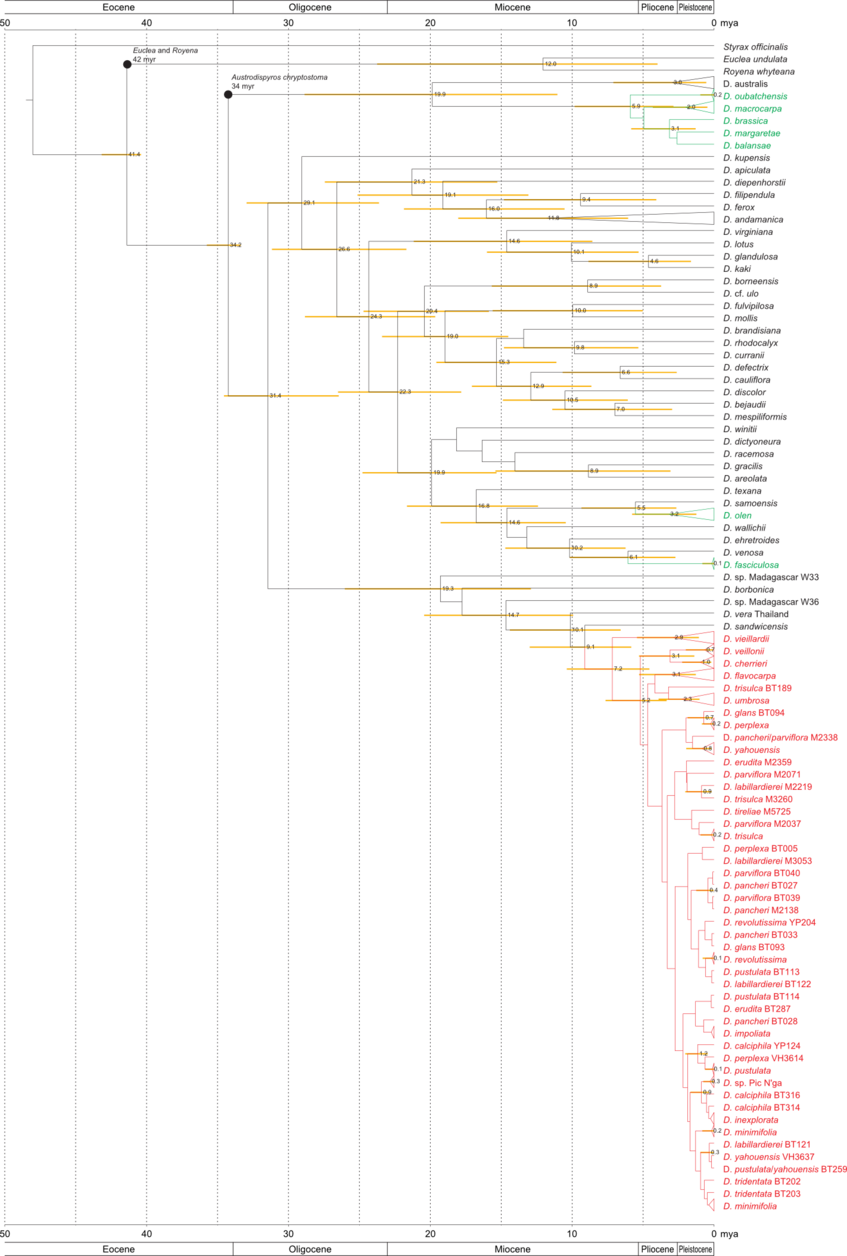
Combined matrix with nodes dated
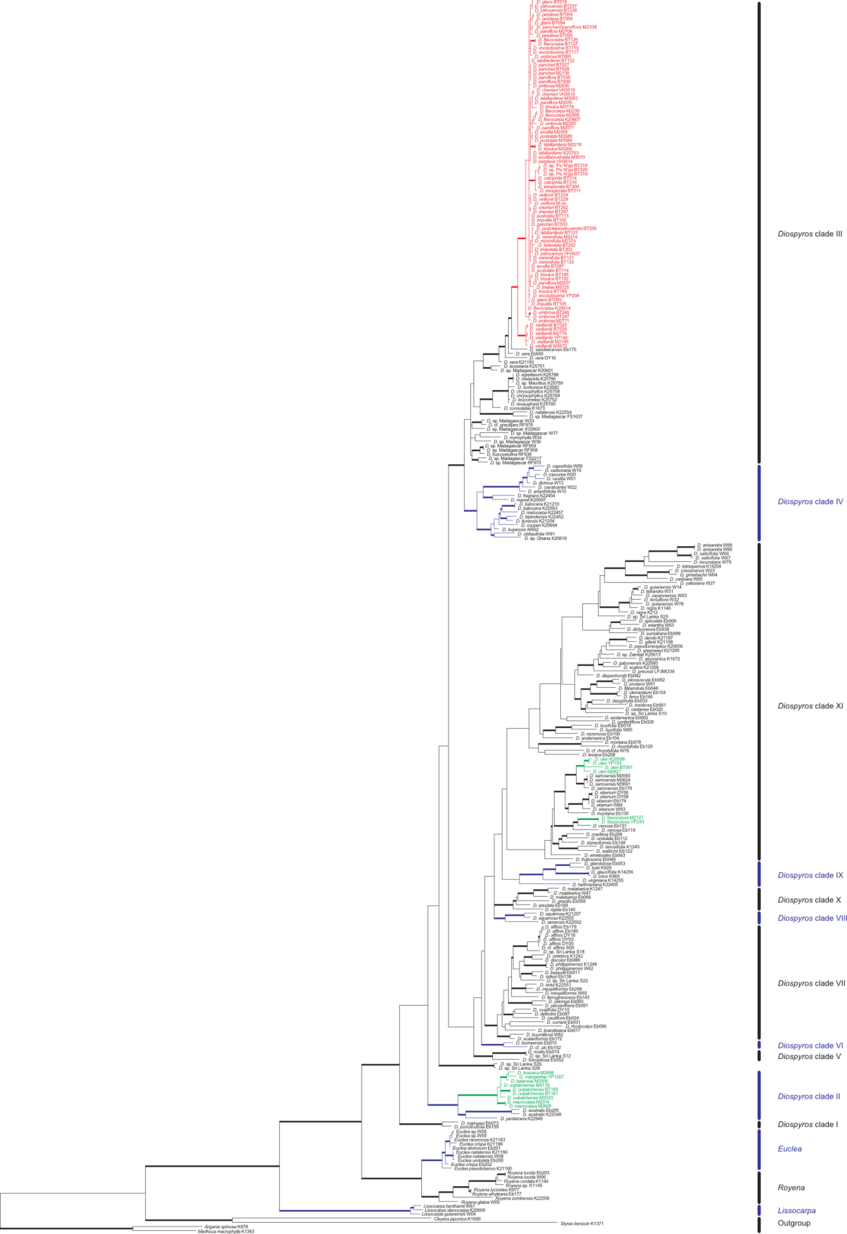
Phylogram from plastid sequences
Species delimitation

D. minimifolia
Species delimitation
Are these rapidly-diverging species really genetically distinct? Is there gene flow between species and has this helped or hindered adaptive radiation? We might expect genetic boundaries between Diospyros species to reflect ecological separation.
To date, we have applied AFLP analysis to our collection of 338 samples (from 71 populations). Restriction site associated DNA (RAD) sequencing and analysis of repeated elements are currently underway.
The figures below are modified from Turner et al. 2013, BMC Evolutionary Biology. (Right-click to enlarge).
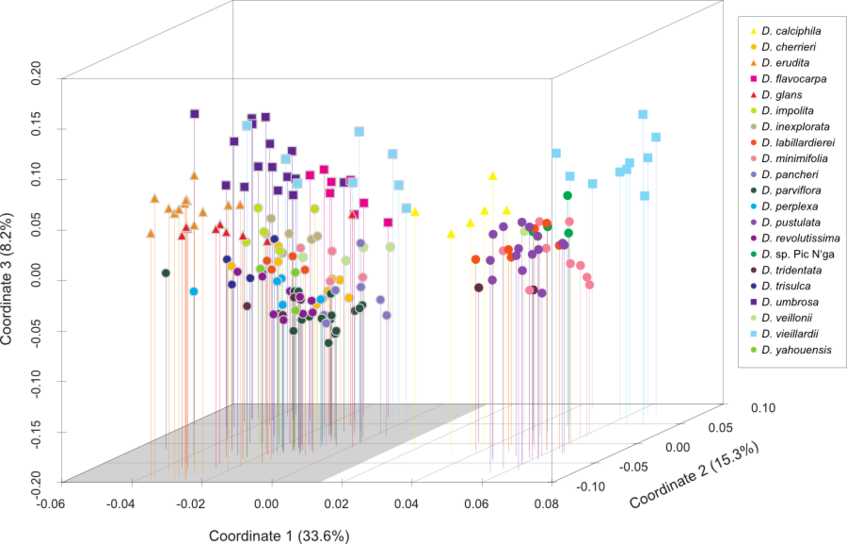
AFLP data: Principle coordinate analysis
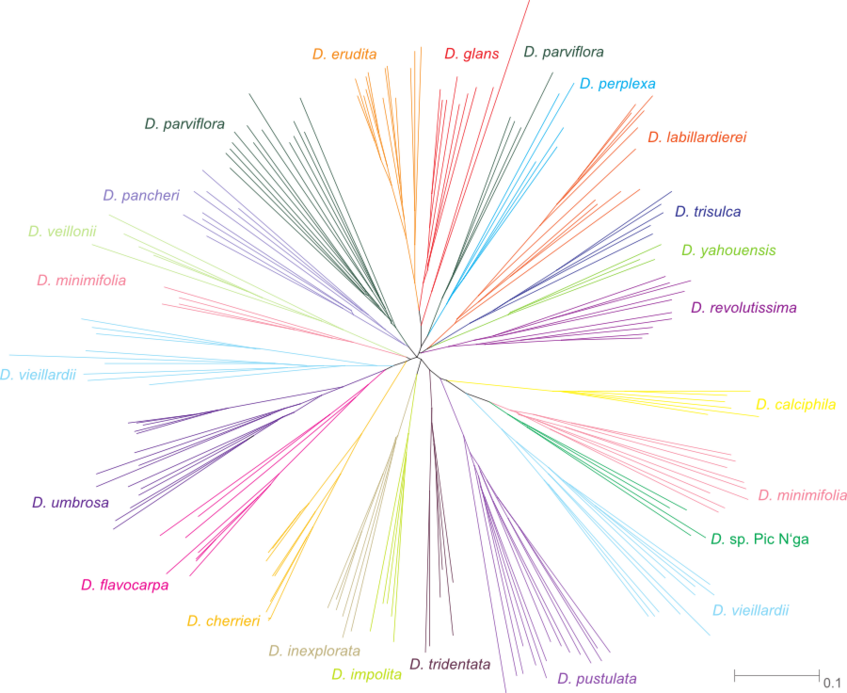
AFLP data: Neighbour joining network
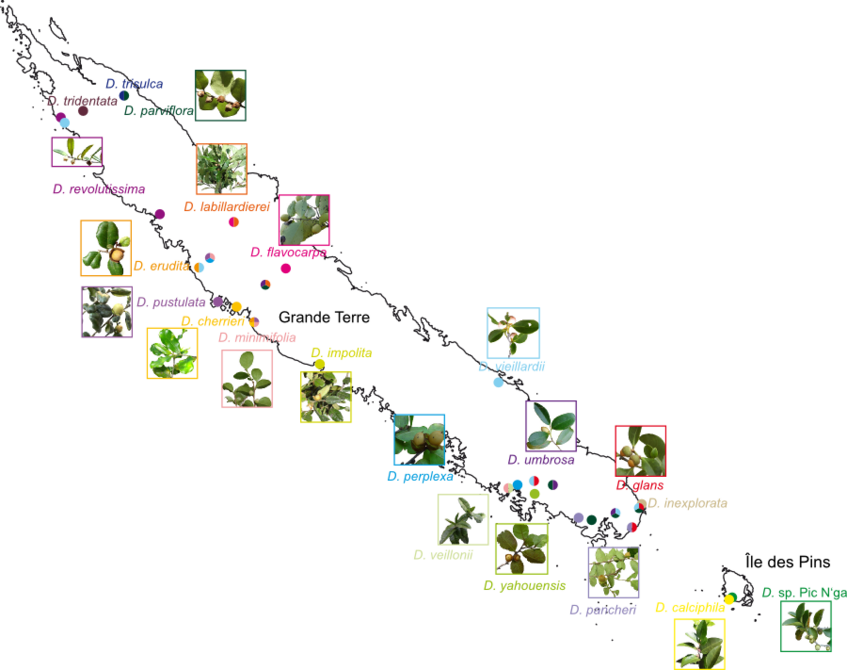
Species matched to collecting localities
Genome size
Rapid speciation might have been accompanied by large-scale changes in genome structure.
Polyploidy is rare in Diospyros, and all of the species examined from New Caledonia have been diploid, with 2n = 30 chromosomes.

D. pustulata
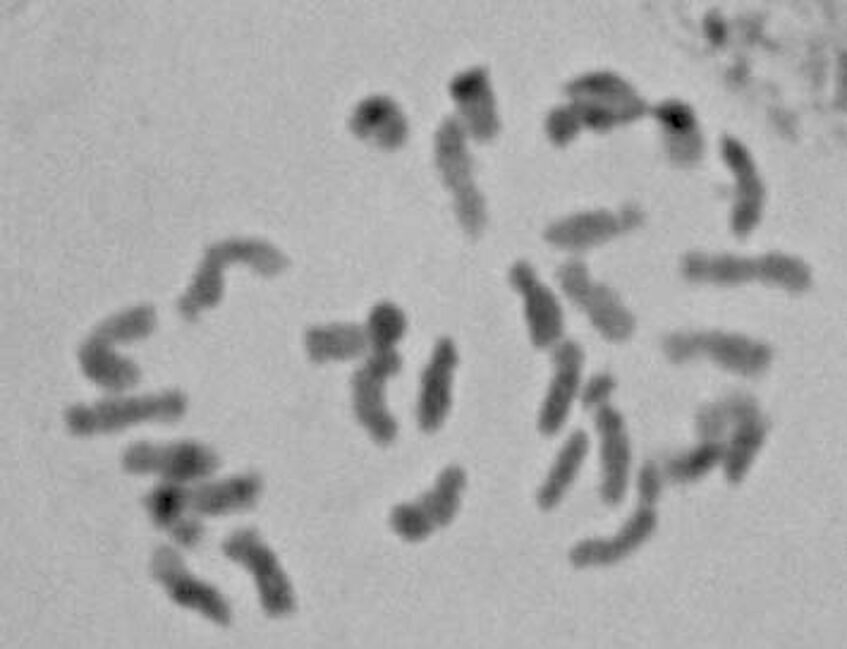
D. pustulata metaphase chromosomes
Despite this we see large variation in genome size, from 0.86 pg in D. olen to 2.3 pg in D. pancheri. Members of Sutee Duangjai's clade III tend to have far larger genomes; this is also the clade that exhibits the greatest diversity and most rapid diversification among New Caledonian species.
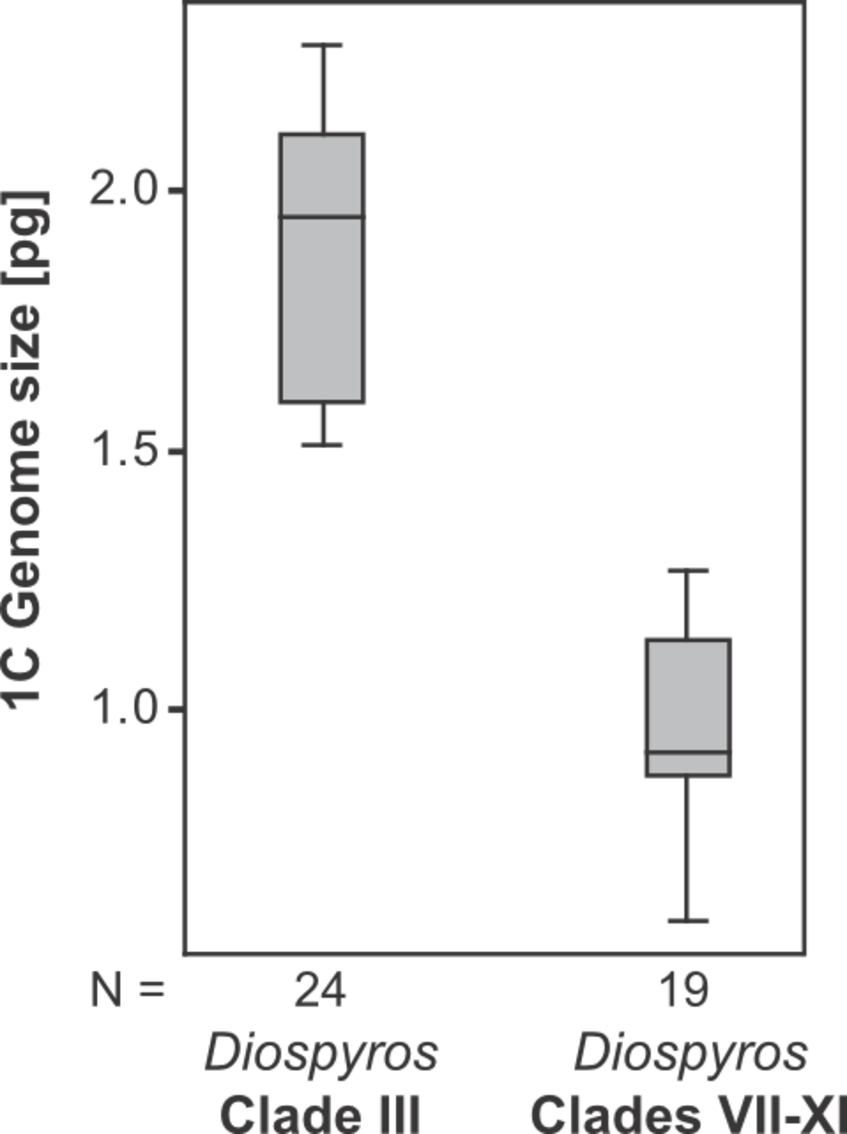
Genome sizes of New Caledonian Diospyros: Clade III vs. VII–XI

Diospyros pancheri
Global Diospyros and Ebenaceae

Male D. gracilis
Global Diospyros and Ebenaceae
Our current research follows on from Sutee Duangjai's doctoral work in Vienna. He studied the phylogenetics of Ebenaceae s.l. using six plastid DNA regions, and recommended a recircumscription of the family into four genera and two subfamilies. He highlighted the importance of both vicariance and long-distance dispersal in shaping the family’s biogeography.
More detailed work on Diospyros followed, and Sutee was able to confirm the monophyly of the genus and analyse biogeographic patterns. New Caledonia emerged as a place where several Diospyros clades had converged and undergone different evolutionary processes. One lineage was paleoendemic of Gondwana origins; another three had established more recently after long distance dispersal from Asia.
The figures below have been published in Sutee's 2006 and 2009 papers. (Right-click to enlarge).
LEGEND:
Left figure: Figure 4 in Duangjai et al., 2006.
Bayesian majority-rule probability tree from analysis of the combined plastid regions. Posterior probabilities are given above branches. Presence of ovary type and number of floral parts, which have been important characters used in Ebenaceae taxonomy in the past, are indicated with symbols to right of the tree. Ovary position can be either superior (black bar) or inferior (white bar). Number of floral parts can be divided into four classes: trimerous flowers (▵), tetramerous flowers (█), pentamerous flowers (*), and hexamerous flowers (○).
Right figure: Figure 4 in Duangjai et al., 2009.
Biogeographical optimization based on dispersal-vicariance analyses performed with the program DIVA (Ronquist, 1996) using one of the 15,000 equally-most parsimonious trees resulting from MP analysis of Ebenaceae. Taxa in red are species from New Caledonia. The distribution of each taxon and the clade number are indicated to the right of the tree. Color legend: Africa and Madagascar; India and Sri Lanka; Australia; New Caledonia; Pacific Islands; Eurasia; South America; Central and North America.
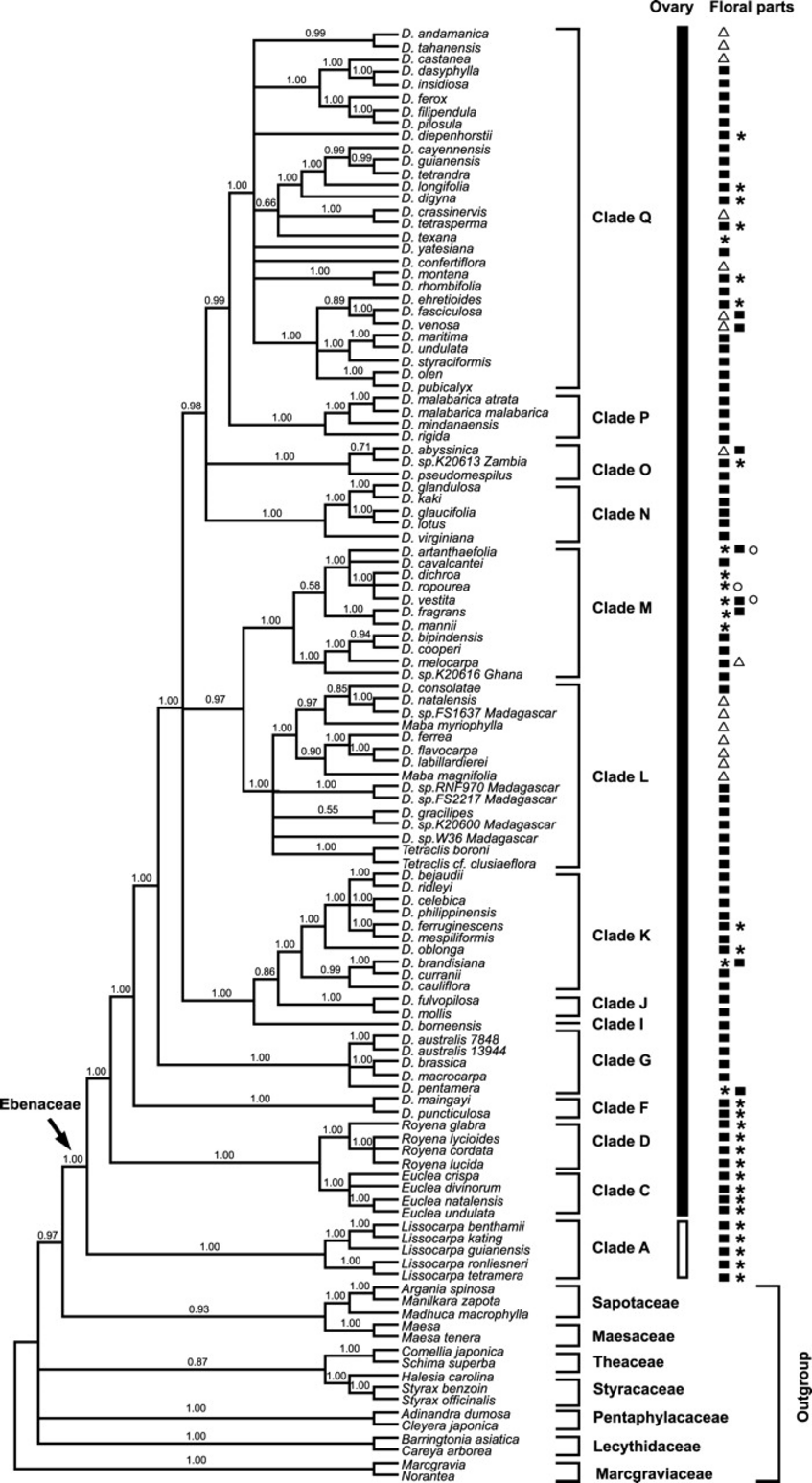
Ebenaceae
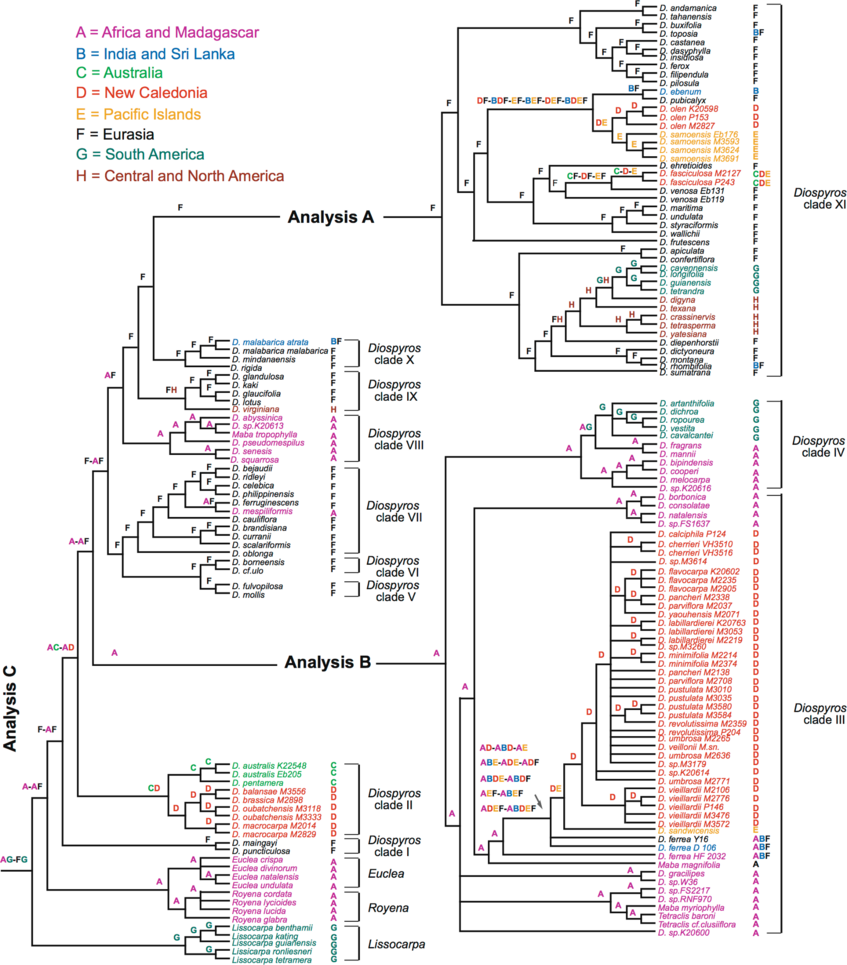
Diospyros biogeography
Fieldwork

To obtain fresh material for DNA extraction, we traveled to New Caledonia from February to April 2011. We collected 338 samples from 71 populations, including 24 out of the 31 New Caledonia species. Of the remaining seven species, material from four was supplied by J. Munzinger from previous expeditions and three have never been found since their first description.
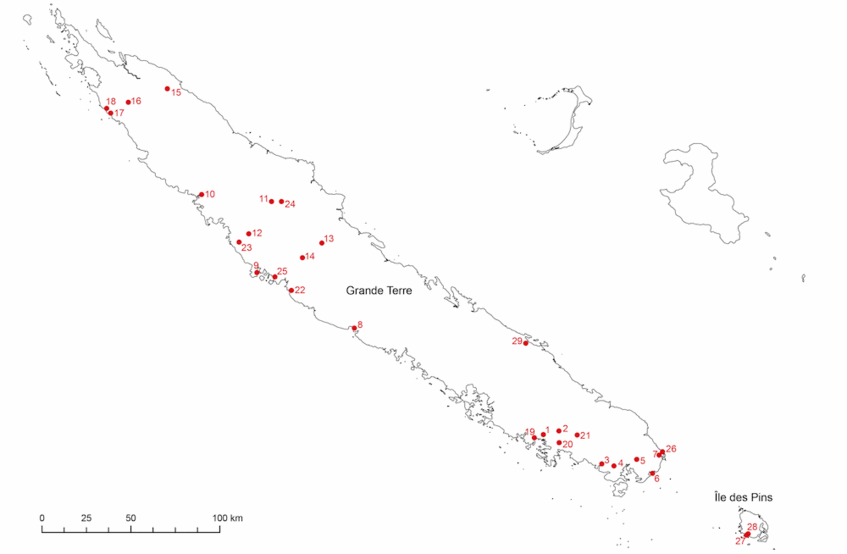
Map of Diospyros collecting localities
- Nakutakoin
- Dumbea
- Between Plum and Prony
- Col de Prony
- Forêt du Grand Kaori
- Port-Boisé
- Kuébini
- La Roche Percée
- Pindai/Népoui
- Voh
- Between Koné and Poindimié
- Conservátoire botanique de Tiéa
- Aoupinié
- Nétéa
- Mandjelia
- Koumac
- Koumac
- Paagouméne
- Baie de Gadji
- Yahoué
- Forêt de la Superbe (Montagne des Sources)
- Moindah
- Plateau de Tiéa
- Plateau de Tango
- Népou
- Ile Kuébini
- Ile des Pins
- Ile des Pins, Pic N'ga
- Thio, Tô De
People
Ao. Univ-Prof. Rose Samuel

Ao. Univ-Prof. Rose Samuel
Principal investigator
Department of Systematic and Evolutionary Botany, University of Vienna
rosabella.samuel@univie.ac.at
Dr. Jérôme Munzinger

Dr. Jérôme Munzinger
Collaborator
Institut de recherche pour le développement, Marseille
Dr. Ovidiu Paun

Dr. Ovidiu Paun
Collaborator
Department of Systematic and Evolutionary Botany, University of Vienna
ovidiu.paun@univie.ac.at
Homepage
Mag. Barbara Turner

Mag. Barbara Turner
Doctoral student
Department of Systematic and Evolutionary Botany, University of Vienna
barbara.turner@univie.ac.at
Dr. Sutee Duangjai

Dr. Sutee Duangjai
Collaborator
Department of Forest Biology, Kasetsart University, Bangkok
Prof. Mark W. Chase

Prof. Mark W. Chase
Collaborator
Jodrell Laboratory, Royal Botanic Gardens, Kew
Publications
Papers
Turner B, Paun O, Munzinger J, Chase MW, Samuel R. 2016. Sequencing of whole plastid genomes and nuclear ribosomal DNA of Diospyros species (Ebenaceae) endemic to New Caledonia: many species, little divergence. Annals of Botany 117: 1175-85.
Paun O, Turner B, Trucchi E, Munzinger J, Chase MW, Samuel R. 2016. Processes Driving the Adaptive Radiation of a Tropical Tree (Diospyros, Ebenaceae) in New Caledonia, a Biodiversity Hotspot. Systematic Biology 65: 212-27.
Turner B, Paun O, Munzinger J, Duangjai S, Chase MW, Samuel R. 2013. Analyses of amplified fragment length polymorphisms (AFLP) indicate rapid radiation of Diospyros species (Ebenaceae) endemic to New Caledonia. BMC Evolutionary Biology 13: 269.
Turner B, Munzinger J, Duangjai S, Temsch EM, Stockenhuber R, Barfuss MHJ, Chase MW, Samuel R. 2013. Molecular phylogenetics of New Caledonian Diospyros (Ebenaceae) using plastid and nuclear markers. Molecular Phylogenetics and Evolution 69: 740–763.
Duangjai S, Samuel R, Munzinger J, Forest F, Wallnöfer B, Barfuss MHJ, Fischer G, & Chase MW. 2009. A multi-locus plastid phylogenetic analysis of the pantropical genus Diospyros (Ebenaceae), with an emphasis on the radiation and biogeographic origins of the New Caledonian endemic species. Molecular Phylogenetics and Evolution 52: 602–620.
Duangjai S, Wallnöfer B, Samuel R, Munzinger J, & Chase MW. 2006. Generic delimitation and relationships in Ebenaceae sensu lato: evidence from six plastid DNA regions. American Journal of Botany 93: 1808–1827.
Conference presentations
Turner B, Paun O, Munzinger J, Duangjai S, Chase MW, Samuel R. 2013. Evolution of New Caledonian Diospyros species (Ebenaceae). Plant Genome Evolution 2013 in Amsterdam, The Netherlands, 8–10 September 2013. [Poster]
Turner B, Munzinger J, Duangjai S, Barfuss MHJ, Wallnöfer B, Paun O, Chase MW, Samuel R. 2012. Diversification of endemic New Caledonian Diospyros (Ebenaceae). 15. Treffen der Österreichischen Botanikerinnen und Botaniker in Innsbruck, Austria, 27–29 September 2012. Berichte des naturwissenschaftlich-medizinischen Vereins in Innsbruck, Supplementum 20.
Turner B, Munzinger J, Duangjai S, Temsch E, Wallnöfer B, Chase MW, Samuel R. 2012. Speciation of New Caledonian Diospyros (Ebenaceae). International Conference on Polyploidy, Hybridization and Biodiversity in Průhonice, Czech Republic, 7–10 May 2012. [Poster]
Samuel R, Turner B, Duangjai S, Munzinger J, Wallnöfer B, Chase MW. 2011. Pattern and mode of speciation of New Caledonian Diospyros (Ebenaceae). IBC2011: XVIII International Botanical Congress in Melbourne, Australia, 23–30 July 2011.
Samuel R, Turner B, Duangjai S, Munzinger J, Wallnöfer B, Chase MW, Barfuss MHJ. 2011. Origin and evolution of New Caledonian Diospyros (Ebenaceae): a phylogenetic approach. IBC2011: XVIII International Botanical Congress in Melbourne, Australia, 23–30 July 2011. [Poster]
Duangjai S, Samuel R, Munzinger J, Wallnöfer B, Forest F, Barfuss MHJ, Chase MW. 2010. Biogeographic origins and evolution of endemic species of Diospyros (Ebenaceae) from New Caledonia: A molecular phylogenetic perspective. 19th International Symposium “Biodiversity and Evolutionary Biology” of the German Botanical Society (DBG) in Vienna, Austria, 16–19 September 2010.
Duangjai S, Samuel R, Wallnoefer B, Munzinger J, Yakandawela D, Chase MW. 2008. A phylogenetic framework for infrageneric classification of Diospyros (Ebenaceae) with an emphasis on a radiation of species in New Caledonia. Botany 2008: Botany without Borders in Vancouver, Canada, 26–30 July 2008.
Duangjai S, Wallnöfer B, Samuel R, Munzinger J, Chase MW. 2006. Phylogenetic relationships and infrafamilial classification of Ebenaceae s.l. based on six plastid markers. Botany 2006: Looking to the future—Conserving the past in Chico, California, USA, 29 July–2 August 2006.
Duangjai S, Samuel R, Wallnöfer B, Chase MW. 2005. Phylogenetic relationships of Ebenaceae: inferred from four regions of plastid DNA and ncp glutamine synthetase. XVII International Botanical Congress in Vienna, Austria, 17–23 July 2005.
